|
|
Kezdőlap-Home
Page |
|
|
II. évfolyam 3. szám 2001. július [HUN] - Magyar cikk
|
Influence of different conditions of electrochemical synthesis on the structure of the deposited refractory compound coatings S.V. Devyatkin Senior scientist (Ph.D.)
1. Introduction Semiconductor and metal-like refractory compounds can be produced by electrochemical synthesis from molten salts in different forms (coating, dendrites or powder). The goal of the present paper is to analyze the influence of different conditions of electrochemical synthesis on the structure of the cathodic product. Electrochemical synthesis of refractory compounds from molten salts is usually performed using 90-95 w% of a supporting electrolyte with 5-10 w% of additional salts consisting of the components of the refractory compound to be deposited. At this low concentration of the electrochemically active species the limiting cathodic current density can be observed on the polarization curves between 0.1 and 1 A/cm2, depending on the concentration of electrochemically active species and other parameters. Depending on the ratio of the applied current density to the limiting cathodic current density (i/io), the following events can take place during electrolysis [1]:
The basic goal of our research was to synthesize protective coatings of different refractory compounds. Baraboshkin [1] singled out the following four main structure types of coherent coatings deposited in an electrochemical way:
The following main factors affect the structure of the cathodic deposits [1]:
The results of Baraboshkin [1] obtained mainly for single metallic deposits, were extrapolated the structure of the synthesized cathodic coatings will be discussed.
2. Experimental Electrolysis experiments were performed in steel containers under an atmosphere of purified argon or CO2. The glassy carbon crucible served as container of salt and anode. Graphite, glassy carbon, Ni, W, Mo, stainless steel was used as cathode materials. Refractory metal borides were deposited from the system NaCl-KCl-NaF-KBF4-K2MeFx (Ti, Zr, Hf – x=6, Nb, Ta - x=7). SiC was deposited from the system Na2CO3-K2CO3-SiO2. Cathodic product were analyzed by X-ray and SEM.
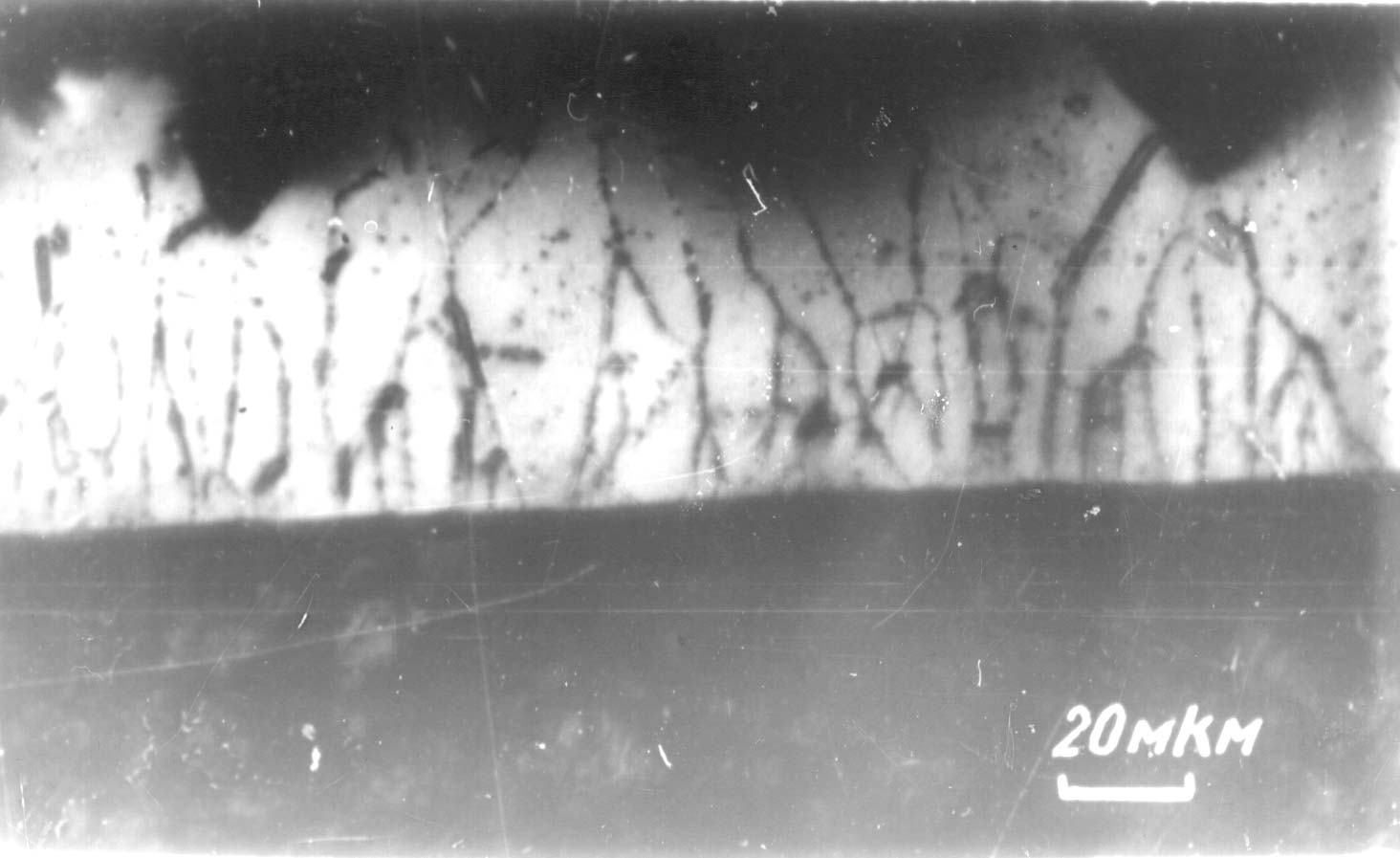
3. The difference between deposition potentials of the two components of the compound to be synthesized The deposition potentials of each component of the compound to be synthesized can be measured in independent preliminary experiments. The absolute value of this difference can be denoted as D E. If this value is compared to the depolarization due to the compound formation (D Edep), the following three cases can be distinguished: Case 1. D E < D Edep: in this case the electrochemical synthesis takes place in one single step. This is characteristic to the deposition of diborides and silicides of titanium, zirconium and hafnium, and of carbides of tungsten, molybdenum and silicon. In this case, usually the coating forms a columnar type structure (as an example see Fig.1. with the deposit of HfB2 [3]). Similar structures as given in Fig.1. were obtained for tungsten carbide [4] and molybdenum carbide [5].
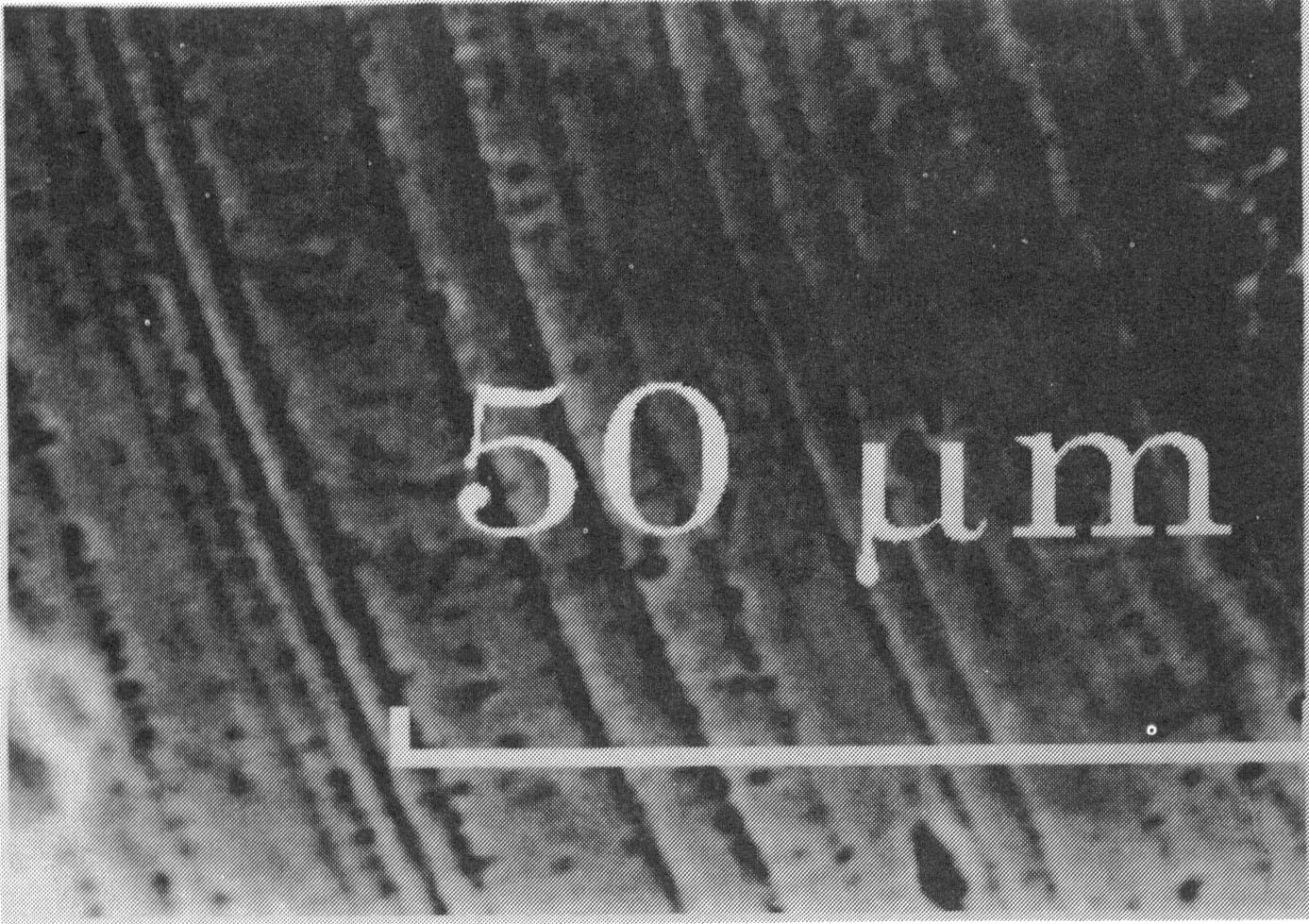
Fig.1. Cross section of a HfB2 coating deposited on a Mo cathode (columnar type deposit) [3] (space bar shows 20 m m) Case 2. D E > D Edep: In this case the electrochemical synthesis is taking place in two steps. First, a component of more positive deposition potential is deposited as a pure phase, and at more negative potentials (or above its limiting current density) the second component is deposited with the formation of the desired compound. Such a two-step mechanism is typical for electrochemical synthesis of borides and silicides of vanadium, niobium, tungsten and molybdenum. In this case the deposition of the coating is possible only at low limiting current density (i.e. at low concentration) of the more electropositive component. If this condition is fulfilled, usually microporous coatings are obtained. In Fig. 2 a typical microporous coating structure is shown, obtained for the niobium diboride coating [6].
Fig.2. Cross section of a NbB2 coating deposited on a steel cathode (a microporous type coating) [6]. Case 3. D E @ D Edep: in this case the potential of electrochemical synthesis coincides with the potential of the component of more positive deposition potential. This is characteristic of the electrochemical synthesis of tantalum diboride. In this case, the layered-type one of the coating structures has been obtained (see Fig. 3) [7, 8]. The formation of this structure has been theoretically explained [2] using the Equilibrium Electrochemical Synthesis (EES) Diagrams [9].
Fig.3. Cross section of a TaB2 coating deposited on a Mo cathode (a layered type structure) [8]
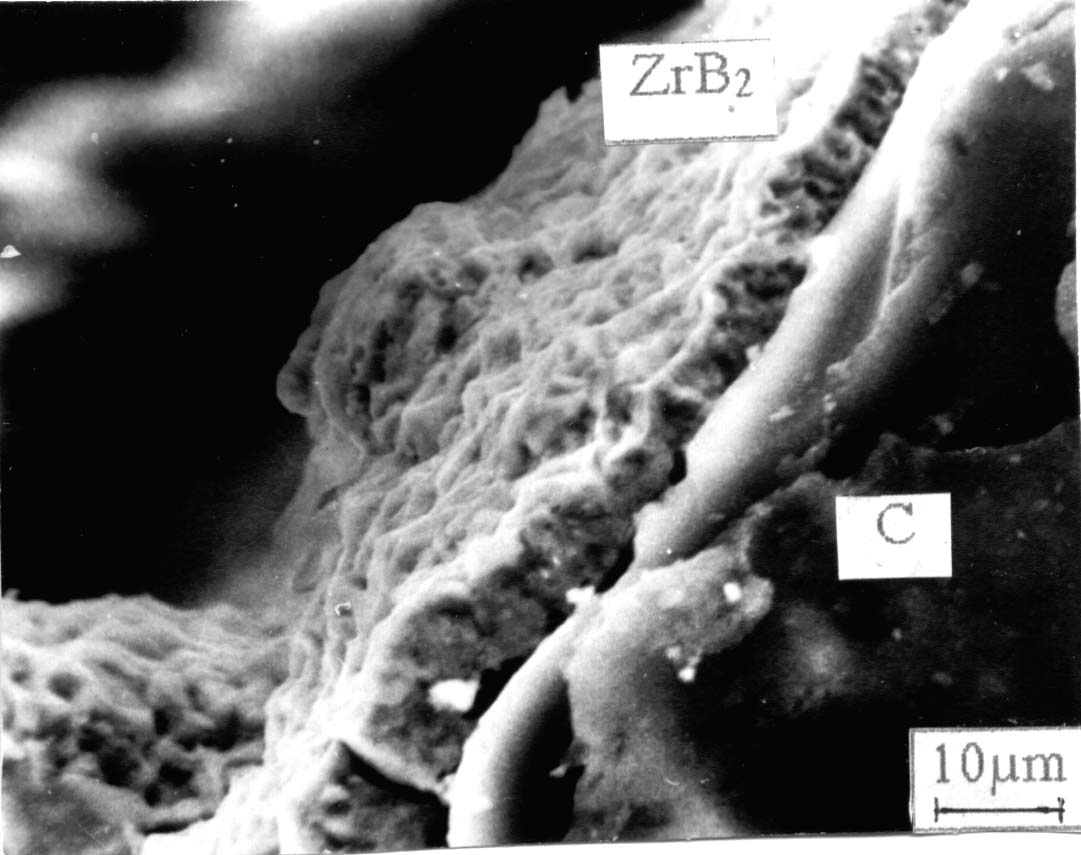
4. Chemical reaction with the formation of an insoluble reaction product If one of the electrochemically active species in the melt participate in a chemical or electrochemical reaction with the formation of an insoluble product, the coating usually has a globular-type structure. Case 1. The electrochemically active component interacts with the product of the cathodic reaction. This is typical for the electrochemical synthesis of zirconium diboride obtained from chloro-fluoride melt, accompanied with the formation of an insoluble zirconium fluoride of lower valence (Fig. 4.) [4].
Fig. 4. Cross section of a ZrB2 coating deposited on cylindrical classy carbon cathode (a globular type coating) [3]. Case 2. One of the electrochemically active species can interact also with the material of the cathode. The example is the electrochemical synthesis of titanium diboride onto carbon cathode from cryolite-alumina melt, containing titanium and boron oxides. In this case an insoluble TiO has been formed as a result of interaction of titanium dioxide with the carbon cathode [10], leading to the formation of a globular-type coating.
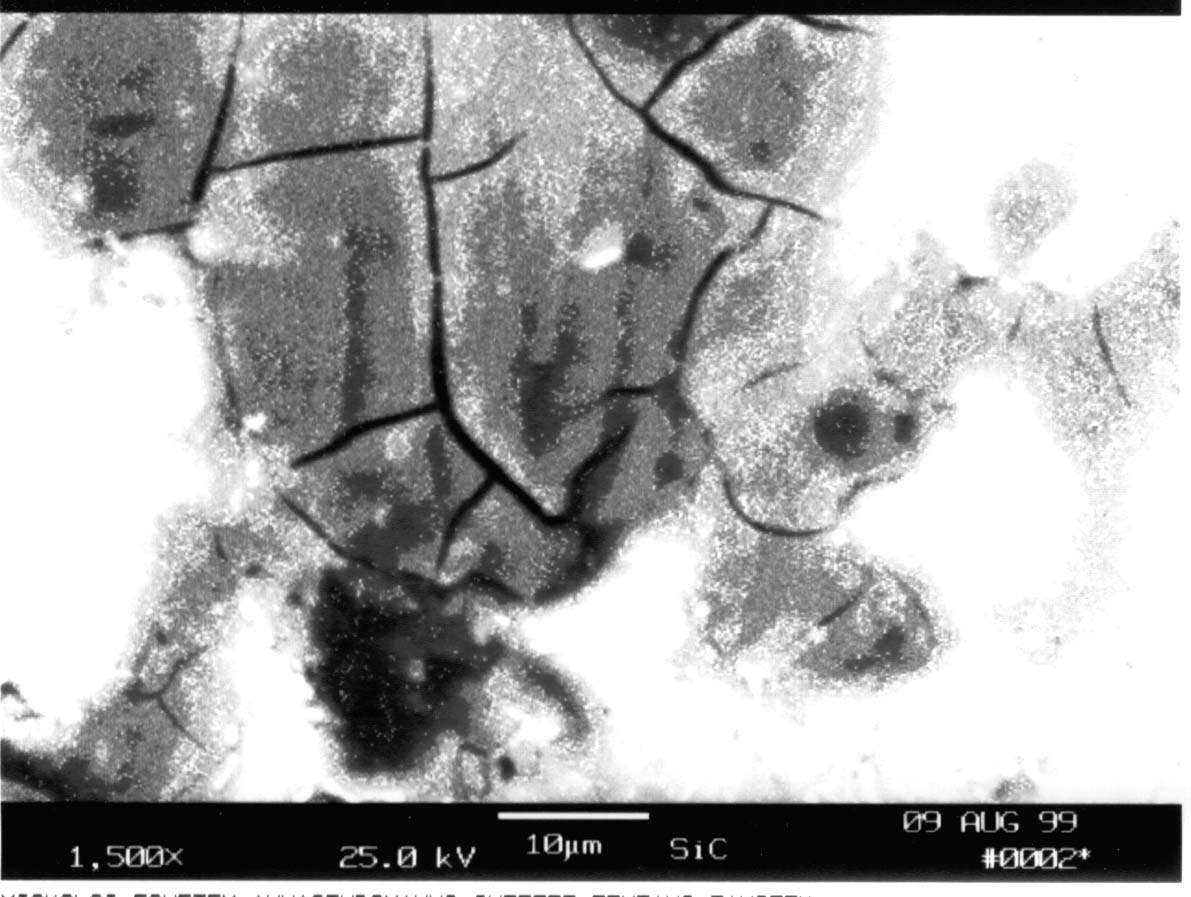
5. Miscellaneous factors The role of the thin cathodic layer: If one of the components of the compound to be deposited can form a stable compound with the material of the cathode, usually a thin sub-layer of this compound is formed between the substrate and the main coating. This sub-layers usually increase the adhesion between the deposit and the substrate. This is the case during deposition of titanium diboride onto nickel cathode, with the formation of a nickel boride sub-layer, ensuring good adhesion between the coating and the material of the cathode [10]. Mechanical mismatch of deposited material and cathode: If the coefficients of thermal expansion of the cathode and the deposited coating differ to a large extent, during cooling the sample from the temperature of the synthesis to room temperature the coating can be cracked, or even separated from the cathode (TiB2 on steel can serve as an example). Electrical resistance of deposited material: In the case deposition of silicon carbide from carbonate-silica melts [11] and synthesis of diamond-like films (with resistance being in the interval of 108-1010 W cm [12]), usually the grain type structure of the coating is obtained (see Fig. 5.).
Fig. 5. Morphology of a SiC coating deposited on steel cathode (a grain type structure of coating) [11].
6. The influence of pulse current plating on the microstructure of the coatings When the coating is synthesised on a cathode, the planar crystallisation front can become irregular and dendrites can grow due to different fluctuations, even if the current density is low. The probablity that these irregularities appear increases with the thickness of the coating. Therefore, thick coatings are hard to obtain. When the pulse technique is used, irregularities can be ‘cut’ and the planar crystallisation front can be maintained for a coating with larger thickness. This method is well known for the electro-crystallisation of metals from water based solutions. The same method was experimentally proven to provide a smoother surface of the TiB2 coating, deposited from a chloro-fluoride melt [13-14].
Conclusions The structure of cathodic deposits is affected during electrochemical synthesis not only by the structure of the substrate, by current density and by temperature, but also by the chemical and electrochemical behaviour of the melt. The different factors discussed in the present paper will help to control better the structure of the cathodic coatings during the electrochemical synthesis of refractory compounds.
References
|
||